Lym-X-Sorb®: Drug Delivery, Fenretinide & Cyclosporine:
Fenretinide:
 Lym-X-Sorb® monomer complexed (in a conceptual model) with a molar equivalent of fenretinide, in yellow.
Lym-X-Sorb® monomer complexed (in a conceptual model) with a molar equivalent of fenretinide, in yellow.
Lym-X-Sorb® solubility and stability:
- Drug is soluble in Lym-X-Sorb® at 1:1 molar ratio.
- Drug in excess of 1:1 molar ratio remains as a solid.
- Lym-X-Sorb®/drug formulations are stable at 40°C for 6 months.
- Drug remains a suspension in corn oil/surfactant formulation.
Oral Bioavailability of Fenretinide:
- Drug in ethanol (soluble): <1% absorption in fed dogs
- Drug in corn oil/surfactant (suspension): Estimate 12-15% absorption in fed dogs or in humans who received a high fat meal.
Lym-X-Sorb®/drug (soluble):
- 70% in fasting dogs
- 90% in fed dogs
- 80-90% in humans who received the same high fat diet as above.
Humans: Comparative Bioavailability of Fenretinamide:
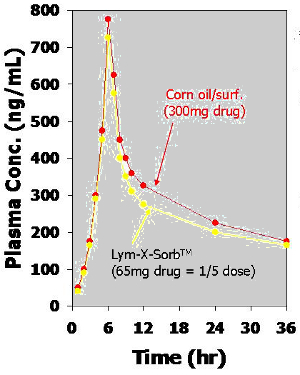
| Pharmacokinetics Parameters: | 65mg Drug/Lym-X-Sorb®: | 300mg Drug/Corn oil/surf: | Ratio (Lym-X-Sorb®/Oil): |
Fenretinamide: |
|||
| CMAX (ng/mL) | 715 | 806 | 4.1 |
| TMAX (hours) | 4.9 | 5.0 | |
| AUC (ng.hr/mL) | 5421 | 7413 | 3.4 |
Metabolite: |
|||
| CMAX (ng/mL) | 258 | 309 | 3.8 |
| TMAX (hours) | 6.3 | 6.1 | |
| AUC (ng.hr/mL) | 4389 | 6011 | 3.4 |
Cyclosporine:
Cyclosporine encapsulated in Lym-X-Sorb® was compared to the current delivery formulations of cyclosporine and administered orally to dogs. The area under the curve showed a 4-5 fold increased absorption of cyclosporine in Lym-X-Sorb® formulation when compared to the commercial formulation. The delayed appearance of drug in plasma for Lym-X-Sorb® formulation indicates that cyclosporine partitions with the chylomicrons into the lymphatic system.
Exclusion Chromatography and Oral Bioavailability of Cyclosporin Graph